|
|
|
SAMPLING
FOR PREFERENTIAL FLOW IN THE UNSATURATED ZONE
Sampling the unsaturated
zone can serve as an early warning system for groundwater contamination.
However, one of the greatest uncertainties in monitoring groundwater
contamination is the possibility that solutes flowing in preferred
paths bypass samplers. Thus scaling point-source measurements to
field-scale estimates may be unreliable and misleading even in the
case when geostatistical methods are employed.
Methods for continuously
sampling solutes in the unsaturated zone involve the collection
of soil water drained by either the force of gravity (e.g., gravity
pan samplers, agricultural tile lines, and shallow wells) or by
applying a "capillary" suction (e.g., porous cup samplers,
wick lysimeters). Whilst all these sampling techniques result in
collection of solutes and water in the unsaturated zone, only tile
lines and pan samplers measure the amount moving to the aquifer.
Gravity pan samplers collect the percolate from a saturated portion
of the soil immediately in contact with the sampler, and thus may
lead to "bypassing" (i.e., solutes and water flowing around
the sampling device).
The figures below (Figures
1a and 1b) illustrate how dramatic differences in sampled concentration
of solutes can occur within a very small volume. Four suction lysimeters
were installed at a depth of 60 cm, and two each at 90 cm, 120 cm,
150 cm and 180 cm depths in a sandy loam near Freeville, New York.
The soil was noted as having worm holes. The depth to groundwater
was 2 m. A 4 cm pulse of bromide, with a concentration of 8000 mg/l,
was applied on first day of the experiment and this was followed
by 4 cm irrigation each day. The suction lysimeters located at 60
cm were affected by preferential flow paths directly connected to
the surface (some induced along the sampling tubes) giving rise
to the widely varying breakthrough curves (Figure 1a). The remaining
sampling devices did not appear to have this problem. Based on the
Convection-Dispersion model, one would expect to see the solute
peaks decrease in concentration with depth. However, as shown in
Figure 1b, the peak concentration observed in the capillary fringe
at 180 cm, for instance, occurred prior to the peak seen at 150
cm, which, in turn, arrived prior to the maximum concentration seen
at the 120 cm depth. This pattern was caused by preferential flow
of water and solutes from the finer soils at the 90 cm depth through
the coarse soil directly to the groundwater table, bypassing the
soil suction lysimeter.
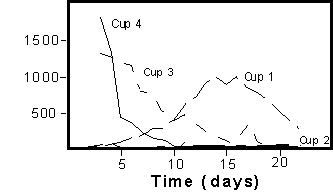
Figure
1a. Breakthrough curves for four porous cup samplers located
at the 60 cm depth
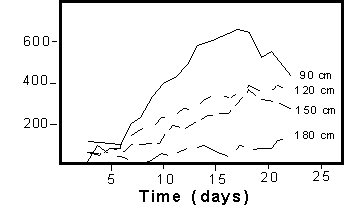
Figure
1b. Breakthrough curves for porous cups at 90, 120,
150, and 180 cm. Each line is the average of two replicates
There are currently two
general approaches, claimed to be independent of the sampling device,
that can be used to sample solute flow in the field. The first is
Bouma's (1990) morphological approach in which a Representative
Elementary Volume (REV) is used. Soil volumes can be considered
"representative" if they are large enough so that individual
flow path differences can be averaged. According to Lauren, et al.
(1988), sampling volumes of 30 peds or more result in "representative"
samples. Thus, REV's characterise "an average flow path"
in which the soil heterogeneities can be studied as a stochastic
or statistical phenomena. However, in situations where concentrations
on the order of parts per billion are considered (e.g. pesticide
transport), then this approach will be inadequate. Rather, individual
preferential pathways that may be responsible for the transport
of chemicals must be sampled. In this case, the approach by Barcelona
and Morrison (1988) to locate the sampling devices in the likely
pathways of water and contaminants is better. When the groundwater
is at shallow depth, then the use of artificial tile lines for sampling
are useful even though the tile lines integrate samples over a field
scale.
 A
modification of the gravity pan sampler is the Alundum tension plate
sampler, in which the percolate is extracted from the unsaturated
soil by suction applied across a alundum filter disc. Fibreglass
wick lysimeters operate by the same principle. The wicks are self-priming
and act as a hanging water column, thus providing a suction to the
unsaturated soil. The wicks are also non-reactive and therefore
can be used to sample solutes. Original wick sampling units consisted
of a single fibreglass wick spread over a 30 cm x 30 cm area. The
disadvantage of this design is that solutes entering the sides of
the sampling unit had to travel a considerable distance to the center,
whilst solutes near the center, flowed without delay, thus giving
rise to large instrument dispersion. This design was improved by
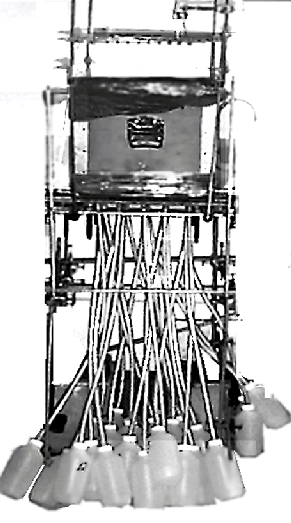
Boll, et al. (1991) and others giving rise to a multi-segment percolation
system shown by Figure 2. These units can be installed in situ in
field sites or alternatively undisturbed soil cores from selected
sites can be extracted and transported to the laboratory for detailed
study. Twenty-five individual fibreglass wicks are placed on a 5
x 5 grid on the basal surface area of the sampling unit (see Figures
3a, 3b, 3c). A
modification of the gravity pan sampler is the Alundum tension plate
sampler, in which the percolate is extracted from the unsaturated
soil by suction applied across a alundum filter disc. Fibreglass
wick lysimeters operate by the same principle. The wicks are self-priming
and act as a hanging water column, thus providing a suction to the
unsaturated soil. The wicks are also non-reactive and therefore
can be used to sample solutes. Original wick sampling units consisted
of a single fibreglass wick spread over a 30 cm x 30 cm area. The
disadvantage of this design is that solutes entering the sides of
the sampling unit had to travel a considerable distance to the center,
whilst solutes near the center, flowed without delay, thus giving
rise to large instrument dispersion. This design was improved by
Boll, et al. (1991) and others giving rise to a multi-segment percolation
system shown by Figure 2. These units can be installed in situ in
field sites or alternatively undisturbed soil cores from selected
sites can be extracted and transported to the laboratory for detailed
study. Twenty-five individual fibreglass wicks are placed on a 5
x 5 grid on the basal surface area of the sampling unit (see Figures
3a, 3b, 3c).
Figure
2. Multi-segment wick percolation system (Deakin University, 1996)
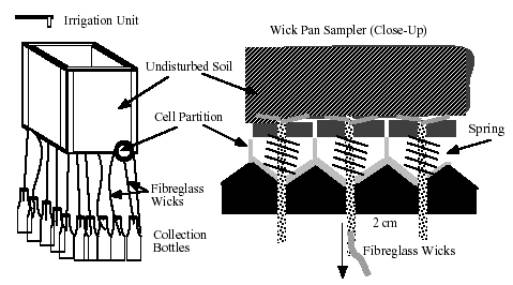
Figure
3a. Schematic view of the multi-wick sampling unit

Figure
3b. Three dimensional view of the alloy-cast base-plate installed
with spring-loaded wick lysimeters prior to mounting on soil
column
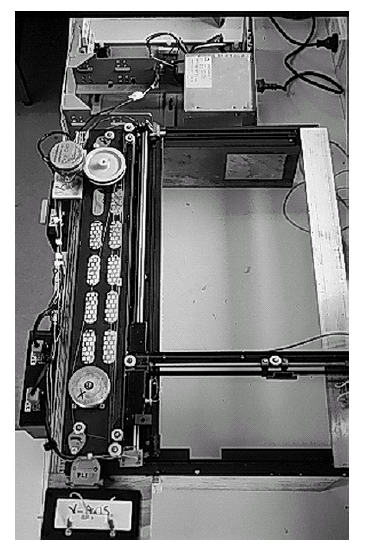
Figure 3c. 12 V
variable velocity, X-Y scanning irrigation unit. The unit
is mounted on top of the soil column and delivers uniform
rainfall. Note that the flux is controlled by peristaltic
pump.
The base-plate
is then firmly pressed against the soil surface by springs. The
length of the wick provides a capillarity equivalent to that found
in the soil and thus can be used to sample unsaturated flow. Being
a porous medium, the wicks have been shown to provide boundary conditions
which mimic those found in the undisturbed soil. For instance, the
capillary force in the wick decreases with increasing flux, thus
eliminating flow - field distortions created by suction-cup lysimeters.
Barcelona, M. J., &
Morrison, R. D. (1988). Sample collection, handling and storage:
Water, soils and aquifer solids. In D. W. Nelson & R. H. Dowdy
(Ed.), Methods for Groundwater Quality Studies, Proceedings of the
National Workshop, Agricultural Research Division, University of
Nebraska, Lincoln, Nebraska. 49 - 62.
Boll, J., Selker, J.
S., Nijssen, B. M., Steenhuis, T. S., Van Winkle, J., & Jolles,
E. (1991). Water quality sampling under preferential flow conditions.
In R. G. Allen, T. A. Howell, W. O. Pruitt, I. A. Walter, &
M. E. Jensens (Ed.), Lysimeters for Evapotranspiration and Environmental
Measures, Proceedings American Society of Civil Engineers , International
Symposium on Lysimetry. New York City. American Society of Civil
Engineers. 290 - 298.
Bouma, J. (1990). Using
morphometric expressions for macropores to improve soil physical
analysis of field soil. Geoderma, 46, 3 - 11.
Lauren, J. G., Wagenet,
R. J., & Wisten, J. H. M. (1988). Variability of saturated hydraulic
conductivity in a Glossaquic Hapludalf with macropores. Soil Science,
145, 20 - 28.
Preferential Flow
|

