Methods of the counties work
ELISA
Enzyme-Linked Immunosorbent Assays (ELISA) provide a convenient screening method for measuring single or closely-related pesticide residues in water. We carry out screening tests at Cornell using commercial ELISA kits which are available for a variety of pesticide active ingredients. Because the analysis is focused on only one or one family of pesticide, these procedures are sensitive to levels down to ~0.05 micrograms per liter (0.05 parts per billion), compared to ~1 microgram per liter sensitivity of the far broader multi-pesticide scans conducted at the DEC pesticide lab as of 2009. As with many tests, these methods can detect pesticides at levels that are too low to reliably quantify, so we report as “trace detections” any results that show a slight response but which are lower than the assay’s lower limit of quantitation (also referred to as the Method Detection Limit), which is typically on the order of 0.1 µg/L (0.1 parts per billion).
A typical test session is as follows:
Fill test tube array, using blanks, standards containing known concentrations, and water samples.
Add antibody solution specific to the pesticide and (in some kits) magnetic particles to each tube. Mix well, then allow time for the antibodies to bind with with pesticide molecules in the standards and samples.
Dump and rinse the bulk liquid from the tubes, halting the binding. The bound antibodies remain inside the tubes. (Kits using magnetic particles employ a powerful magnet tray to retain the bound antibodies in the tubes when washing.)
Add a color-developing solution that binds with the bound antibodies.
Use a spectrophotometer (which projects light of a specific wavelength through the test tubes) to measure the extent of light absorption in each sample. The concentration in each sample can be derived mathematically by fitting a curve that relates the light absorption levels of standards containing with known concentrations of the pesticide being tested.
We analyze all samples and standards in duplicate, using arithmetic means of the duplicates to check for excessive variability. Test kit protocols specify quality assurance criteria that we evaluate to ensure that our test results are reliable.
The kits used most often have been from Modern Water (who purchased Strategic Diagnostics). Abraxis kits have also been used.
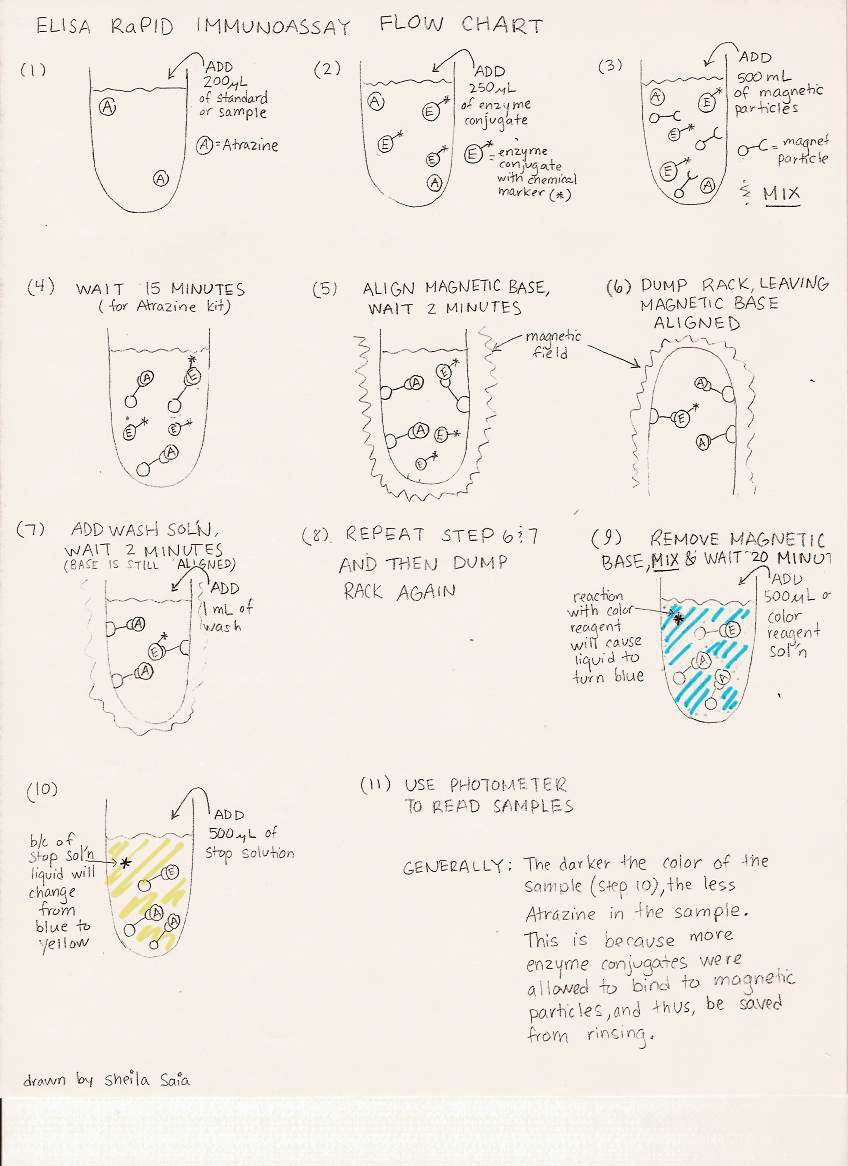
ELISA method in a graphic, drawn by grad student Sheila Saia
DEC Laboratory Pesticide Scans
The DEC established a pesticide specialty laboratory in Rensselaer shortly before our research began in 2003, and equipment upgrades have substantially improved the detection limits for a broad range of currently-used pesticides and herbicides. As of 2019 the laboratory employs Ultra Performance Liquid Chromatography (UPLC) and mass spectrometry (MS) to test our samples for around 50 pesticide compounds. Analyses and method detection limits (MDL) for the NYS DEC laboratory for the round 5 Wayne County samples are shown below, with all MDL concentrations reported as micrograms/Liter (parts per billion).
Method codes are: U – UPLC/MS-MS; G – GC/SIM-MS
| Analyte | MDL | Code | Analyte | MDL | Code |
| 2,4-D | 1 | U | Imazalil | 1 | U |
| 3 Hydroxy Carbofuran | 1 | U | Imidacloprid | 1 | U |
| 3,4,5 Trimethacarb | 1 | U | Isoproturon | 1 | U |
| 6-chloro-4-hydroxy-3-phenyl-pyridazin | 1 | U | Isoxaflutole | 1 | U |
| Acephate | 1 | U | Linuron | 1 | U |
| Aldicarb+Methomyl | 0.35 | U | Malathion | 1 | U |
| Aldicarb Sulfone | 1 | U | MCPA | 0.44 | U |
| Aldicarb Sulfoxide | 1 | U | MCPP | 1 | U |
| Amidosulfuron | 1 | U | Metalaxyl | 1 | U |
| Atrazine | 1 | U | Metamitron | 1 | U |
| Azinphos Methyl | 1 | U | Methamidophos | 1 | U |
| Azoxystrobin | 1 | U | Methiocarb | 1 | U |
| Bendiocarb | 1 | U | Metolachlor | 1 | U |
| Benfluralin | 1 | G | Metsulfuron-Methyl | 1 | U |
| Butocarboxim | 1 | U | Monocrotophos | 1 | U |
| Butoxycarboxim | 1 | U | Nicosulfuron (Accent) | 1 | U |
| Carbaryl | 1 | U | Omethoate | 1 | U |
| Carbendazim | 1 | U | Oxamyl | 1 | U |
| Carbofuran | 1 | U | Oxydemeton-Methyl | 1 | U |
| Chlorosulfuron | 1 | U | Pendimethalin | 1 | U |
| Chlorpyrifos | 1 | G | Primicarb | 1 | U |
| Cinosulfuron | 1 | U | Promecarb | 1 | U |
| Clethodim | 1 | U | Propamocarb | 1 | U |
| Clopyralid | 1 | U | Propoxur | 1 | U |
| Cyprodinil | 1 | U | Prosulfuron | 1 | U |
| Daminozid | 1 | U | Pymetrozine | 1 | U |
| DCPP | 1 | U | Pyridate | 1 | U |
| Demeton-S-Methyl Sulfone | 1 | U | Pyrimethanil | 1 | U |
| Diazinon | 0.7 | U | Quinmorac | 1 | U |
| Dicamba | 0.44 | U | Quizalofop Ethyl | 1 | U |
| Dimethoate | 1 | U | Rimsulfuron | 1 | U |
| Dithiopyr | 1 | G | Spiroxamine | 1 | U |
| Diuron | 1 | U | Tebuconazole (Folicur) | 1 | U |
| Ethiofencarb | 1 | U | Tebufenozide | 1 | U |
| Ethiofencarb-sulfone | 1 | U | Thiacloprid | 1 | U |
| Ethiofencarb-sulfoxide | 1 | U | Thifensulfuron-Methyl (Pinnacle) | 1 | U |
| Fenhexamid | 1 | U | Thiodicarb | 1 | U |
| Fenoxycarb | 1 | U | Thiofanox-sulfone | 1 | U |
| Fenpropimorph | 1 | U | Thiofanox-sulfoxide | 1 | U |
| Flazasulfuron | 1 | U | Triademefon | 1 | U |
| Fluazifop-p-butyl | 1 | U | Triasulfuron | 1 | U |
| Flufenoxuron | 1 | U | Trichlorfon | 1 | U |
| Furathiocarb | 1 | U | Triclopyr | 1 | U |
| Halofenozide | 1 | U | Trifluralin | 1 | G |
| Haloxyfop Ethoxyethyl | 1 | U | Triflusulfuron-Methyl | 1 | U |
| Haloxyfop Methyl | 1 | U | Vamidothion | 1 | U |
Ion Chromatography for Nitrate
Ion chromatography measures the concentration of ions (such as nitrate, sulfate and phosphate) in water, using a sorbing column to separate each of the ions prior to measuring their concentrations using a conductivity detector.
The procedure for ion chromatography in our particular Dionex equipment is as follows:
Prepare standards, blanks, and samples in specialized 0.5 mL or 2 mL vials. Samples may be diluted with a known amount of deionized water if expected concentrations of sulfate are too high.
The ion chromatograph (IC) has an autosampler that injects the sample into the flow stream through the separation column and then the detector, which records specific conductance over time. It requires about 20 minutes to automatically process each vial.
IC control software computes a linear calibration equation, relating integrated specific conductance in the standards to their known concentrations. The analyzer then applies this calibration to the results from the samples and reports the computed concentrations of these ions.
Since 2008 we have used this method to test for nitrate, chloride, and sulfate. High sulfate levels tend to interfere with the determination of low levels of nitrate, and in some counties we have improved our results by using 10-fold dilutions to reduce sulfate interference.
Sample Collection and Storage
Samples are taken before the water has passed through any site-specific treatment (such as softening, chlorination or sediment filters), whenever possible. (Treatment at private wells is rare in NY, thus a sample after treatment is unrepresentative of most other wells in an area.) The sampling point is often an outdoor or basement tap, or a cold water tap indoors.
Samples are collected and stored following these steps:
Label certified pre-cleaned HDPE plastic bottles with a location code and date. (To maintain confidentiality, only Cornell project personnel and in some cases local cooperators know the identity and location linked to each code.)
Allow the water to run for several minutes to empty any equalization tank and draw water that has not been standing in the well for too long. Rinse each bottle three times with the water being sampled, then fill to the shoulder, allowing some air space for expansion when frozen.
Transport samples back to Cornell in ice chests, and freeze until analysis.
Frozen samples are shipped to DEC in heavily insulated ice chests, with bubble wrap for cushioning and extra insulation. Shipping is via an express carrier to limit chances for samples to thaw.
During analysis at Cornell, samples are thawed at room temperature. For ELISA work, the sample must reach room temperature. Samples are returned to refrigeration (if there will be more analysis on the following day), and eventually to a freezer.
None of the pesticides being tested for at Cornell or NYS DEC is likely to volatilize from pumped ground water at a rate high enough to affect concentrations during brief handling, thus the handling protocol does not protect against loss of volatiles.
Cornell collects extra bottles during sampling to be able to reship or carry out additional tests without resampling. Samples are stored frozen for several years in case any reanalysis is needed. DEC tests indicate good stability of frozen samples spiked with selected pesticides.
Last updated 2023-09-21 sp17 AT Cornell.edu.